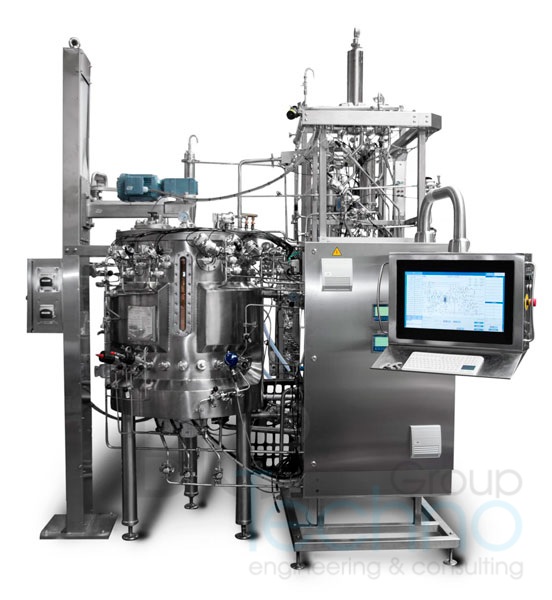
In the current stage of COVID-19 global pandemic many pharmaceutical companies are faced with a problem of large-scale vaccine manufacturing. Increased worldwide demand for technological equipment more than doubled estimated delivery time among almost all European and American manufacturers.
As a manufacturer of bioprocessing equipment and automation services, Biotechno uses our expertise and SmartFrame® engineering technology to provide our customers with shortest delivery time possible, seamless installation and startup services and fast troubleshooting with up to 85% remote resolution. Biotechno gives any customer involved with COVID-19 vaccine manufacturing special delivery plan customized to ensure that you will get the equipment you need in time.
Biotechno COVID-19 manufacturing plan includes: Biotechno COVID-19 manufacturing plan includes:
Fill the form below to request a quote or contact us at: office@sysbiotech.at